Background:
Immune thrombocytopenia (ITP) is an autoimmune disease mediated by anti-platelet autoantibodies. Helicobacter pylori ( H. pylori) may modulate the Fcγ-receptor balance of monocytes/macrophages in favor of activating Fcγ receptors, and its components may mimic the molecular makeup of platelet antigens. In Mexico, the percentage of seroprevalence is 66%. There is not enough evidence available to determine the impact of the screening and eradication of H. pylori in ITP patients in the context of a region with a high prevalence of this infection.
Study design and methods:
This retrospective cohort study included all adult patients older than 18 years, newly diagnosed with ITP in a reference center in Mexico City. We evaluated the frequency and impact of H. pylori screening and eradication in patients diagnosed between 2016 and 2023. The H. pylori screening was made by urea breath test. Response criteria were based on the 2019-American Society of Hematology guidelines.
Results:
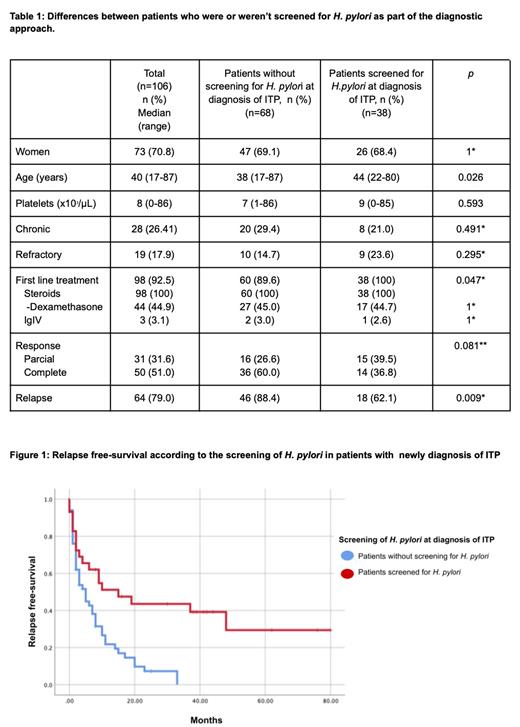
After a median follow-up of 38 months (interquartile range (IQR) 16.7 - 58.2 months), 106 patients with ITP were identified, of whom 73 (69%) were women. Most of them (92.3%) required first line treatment, all with corticosteroids: 44.9% dexamethasone, 44.9% prednisone, and 10.2% methylprednisolone followed by prednisone. Only 3 patients (3%) received intravenous immune globulin. Overall response rate (ORR) was observed in 76% with complete response (CR) of 47%, and partial response (PR) of 29%. During follow-up, 28 patients (26.5%) developed chronic ITP. Screening for H. pylori was performed in 82 patients (77.5%): 35.8% at diagnosis, 21.9% at relapse and 19.8% at a different time point. A comparison was made between the patients who underwent H. pylori test at diagnosis (N=38) vs. those who didn't (N=67) and the only baseline difference was a younger age in patients untested: 38 vs. 44 years (p=0.026) (see Table 1). Only 2/38 patients tested for H. pylori at diagnosis had gastrointestinal symptoms and both were negative for H. pylori. All the patients with a positive result for H. pylori infection received treatment and in all the patients eradication was confirmed. There was no significant difference in responses to the first line treatment between patients with and without H. Pylori infection: CR 30.0% vs. 44.4%, PR 50.0% vs. 27.7% and non-response 20.0% vs. 27.7% (p=0.387). Patients who underwent an H. Pylori test at diagnosis and eradication treatment if positive, had a lower relapse rate: 88.4% vs. 62.1%, p=0.009 (OR 0.213; 95% CI 0.069-0.664). Median relapse free survival was higher in patients who were screened for H pylori at diagnosis vs. those who were not: 15 months (95% CI 2.1-27.9 months) vs. 5 months (95% CI 2.5-7.5 months), p=0.001) (see Figure 1).
Conclusions:
These results suggest that screening and eradication of H pylori since diagnosis can be associated with a lower risk of relapse and a higher relapse-free survival in the context of a country with a high-prevalence of H. pylori infection. We found no relation with the presence of H. pylori and the platelet count or gastrointestinal symptoms, so the screening should be considered regardless of these aspects. The impact of treating H. pylori on ITP-responses cannot be assessed because all the patients receive eradication treatment, but the strategy of screening and treating if positive is associated with lower relapse-rates.
Disclosures
Orozco Collazo:Janssen: Consultancy, Honoraria. Demichelis:AMGEN: Consultancy, Honoraria; Abbvie: Honoraria; Pfizer: Consultancy, Honoraria; TEVA: Consultancy, Honoraria; Astellas: Consultancy, Honoraria.